- Home
- Member Resources
- Pathology Case Challenge
- Pasteurella
Clinical Summary
A 62-year-old woman presents to the emergency department complaining of three days of subjective fevers and chills. Her initial evaluation was notable for both a fever of 100.4°F (38°C) and an 8 cm-wide warm, erythematous, indurated rash on the right calf. The borders of this lesion were ill-defined. On palpation, the rash was tender and without any notable crepitus. The rest of the physical exam was unrevealing. Notably, her white blood cell count (WBC) was elevated to 17,900/µL (normal: 4,000-11,000/µL) with a differential of 86.8% neutrophils (normal: 50-70%) and 8.1% lymphocytes (normal: 18-42%). All other laboratory parameters were within normal limits. Following her emergency department evaluation, the patient is admitted to the hospital in stable condition.
Master List
- streptococci
- Staphylococcus aureus
- E. coli
- Neisseria spp.
- Moraxella spp.
- Klebsiella spp.
- Pasteurella multocida
- Fusobacterium spp.
- Enterobacter spp.
- Porphryomonas spp.
- Pseudomonas aeruginosa
- Bartonella henselae
Archive Case and Diagnosis
This material was originally released as 2015 CPIP J Case 10: Microbiology - A Classic Case of Pasteurella multocida.
Criteria for Diagnosis and Comments
Cases of cellulitis and deeper soft tissue infections are attributed to breaching of the protective skin barrier by bacterial organisms. In these contexts, the most frequently encountered organisms are β-hemolytic Streptococci (eg, Streptococcus pyogenes) and Staphylococcus aureus1. These virulent pathogens frequently colonize the skin, and are thus responsible for an overwhelming majority of cellulitis cases. Other etiologic agents are much less common and generally associated with specific exposures and modes of trauma.
This patient's story of a cat bite/scratch directly preceding the onset of symptoms raised concerns for infection with zoonotic pathogens associated with cats. Bite wound infections are often polymicrobial reflecting the animal’s normal oral/ginigival flora. The organisms most commonly recovered from cat bite wounds include:
- Pasteurella multocida (75%)
- streptococci (46%)
- S. aureus (35%)
- Neisseria spp. (35%)
- Moraxella spp. (35%)
- Fusobacterium spp. (33%)
- Porphryomonas (30%) (24)
| Image 1 | Image 2 |
 |
 |
| Image 3 | Image 4 |
 |
 |
Dog bites are less likely to become infected than cat bites (5% vs 80%). Organisms recovered in culture of dog bite wounds include:
- streptococci (46%)
- S. aureus (46%)
- Neisseria (32%)
- Fusobacterium (32%)
- Pasteurella canis (26%)
- P. multocida (22%) (24)
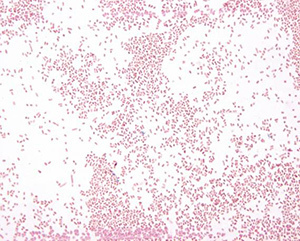
Gram stain of the positive blood culture broth demonstrated Gram-negative organisms with a distinct cocco-bacillary or small rod forms (Image 1). Organisms with this Gram stain appearance are somewhat unusual. The most common Gram-negative blood stream isolates, Enterobacteriaceae organisms (E. coli, Klebsiella, Enterobacter, etc) and Pseudomonas, have a more distinctly rod-like Gram stain appearance and are larger in size. Haemophilus is coccobacillary but rarely isolated from blood. The even rarer blood isolates of Francisella tularensis and Brucella spp. are distinctly minute coccobacillary organisms, associated with very specific zoonotic and environmental exposures.
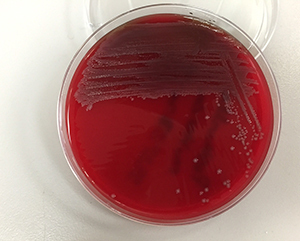
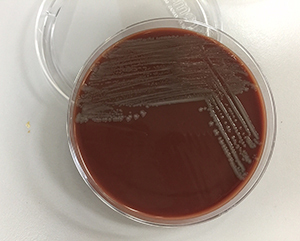
It is also notable that this organism grew well on both Blood (Image 2) and Chocolate agars (Image 3) but not MacConkey agar (Image 4). The selectivity of MacConkey agar is attributed to the bile salts and crystal violet mixed into the agar. These components inhibit the growth of Gram-positive bacteria, while simultaneously permitting the growth of most Gram-negative bacteria2. The most common Gram-negatives isolated from human infections are Enterobacteriaceae, with Escherichia coli being the most frequently encountered in cases of urinary tract infections and bacteremia3. Enterobacteriaceae flourish in environments that are normally bile-rich such as the lower gastrointestinal tract, and as such are not inhibited by the presence of bile salts in the agar. The absence of growth on MacConkey agar suggests that the organism from this patient is not one of the typically encountered members of the Enterobacteriaceae family.
Pasteurella infections (with P. multocida or other Pasteurella spp.) have a wide range of clinical presentations. Skin and soft tissue infections, as in this patient's case, are the most common. These infections are usually associated with cat bites, and to a lesser extent with dog bites. A study by Francis and associates published in 1975 reported that out of 100 cases of Pasteurella multocida isolated from pet-inflicted wounds, 76% were linked to cat bites and the remaining 24% to dog bites4. Localized cellulitis with erythema, induration, and tenderness occurs at the site of inoculation and can progress rapidly. If superficial wounds are not properly treated, deeper tissues can become infected with abscess formation. Cases of Pasteurella multocida osteomyelitis have also been reported as a result of direct extension from initially superficial wounds5. Cases of septic arthritis caused by Pasteurella multocida, whether by direct extension or hematogenous spread, have also infrequently been reported6.
Both upper and lower respiratory tract infections can also be caused by Pasteurella species. The clinical presentation is often varied and non-specific, including sinusitis, bronchitis, and pneumonia. The severity of symptoms also varies greatly, and lower respiratory infections can progress to frank empyema. There is usually an underlying airway disease in affected patients, most often chronic bronchitis, bronchiectasis, chronic obstructive pulmonary disease, or another antecedent respiratory infectious process7, 8.
Overall, bacteremia is an uncommon initial presentation for Pasteurella infection. However, as in this patient's case, secondary bacteremia can develop9. Indeed, almost all cases of Pasteurella bacteremia can be traced back to some other primary infection. In rare instances, blood-borne Pasteurella can seed native or prosthetic cardiac valves resulting in infectious endocarditis10.
There are also a myriad of rarely encountered presentations of Pasteurella infection including meningitis, endophthalmitis, and peritonitis11,12,13. In some instances, the history of potential exposure to Pasteurella is unclear, though secondary dissemination of the organism from some occult site of inoculation is the presumed route of infection.
The populations most at risk for developing Pasteurella infections are, not surprisingly, those that are frequently exposed to animals, domesticated or otherwise - farmers, animal breeders, veterinarians, abattoir workers, and owners of common household pets. However, in some infectious cases there may not be a definitive source of exposure. As previously mentioned, there may or may not be pre-existing medical conditions that predispose to certain types or sequelae of Pasteurella infection (eg, dermatologic, pulmonary).
Interestingly, despite being a Gram-negative organism, Pasteurella species are almost always susceptible to penicillin14. Therefore, in straightforward cases of infection, oral penicillin is often the preferred anti-microbial for treatment once the organism has been identified. Less frequently, cases of penicillin-resistant, β-lactamase-producing Pasteurella species have been encountered, particularly in respiratory isolates15; however, the addition of β-lactamase-inhibitors to a penicillin drug (eg, amoxacillin-clavulanic acid, ampicillin-sulbactam, piperacillin-tazobactam) is reported to be efficacious against such strains. Due to this advantage, such β-lactam-β-lactamase inhibitor combinations are the recommended empiric anti-microbial therapy for cat bites since up to 80% can become infected. Furthermore, since co-infection with organisms such as Staphylococcus aureus are not infrequent, such combination drugs provide added coverage in most cases16.
Pasteurella species are generally susceptible to fluoroquinolones as well, and can also be used for initial, empiric broad-spectrum anti-microbial therapy and continued if there is evidence of response to treatment. Alternative choices for treatment include broad-spectrum (later generation) cephalosporin drugs and tetracyclines such as doxycycline 17. Pasteurella spp. are often resistant to first-generation cephalosporins, erythromycin, and clindamycin18. There are rare reports of tetracycline resistance.
Susceptibility testing can be performed to further define therapeutic options in more serious cases. Specifically, the Clinical and Laboratory Standards Institute (CLSI) defines both disk diffusion and broth microdilution minimal inhibitor concentration (MIC) interpretive criteria for a number of antibiotics in their guidance document for susceptibility testing of fastidious and infrequently isolated bacteria19. In most instances, however, routine susceptibility testing of Pasteurella isolates is not always necessary for patient management. The more common isolates from animal wounds (including Pasteurella) may simply be treated empirically, also with the understanding that the infection may be polymicrobial. In contrast, susceptibility testing of isolates from otherwise sterile sites (eg, blood cultures) and some respiratory infections may be warranted in order to optimize targeted treatment.
Supplementary Questions:
- Pasteurella spp. are typically susceptible to which class of antibiotics?
- Beta-lactam/beta-lactamase inhibitor combinations
- Erythromycin
- Clindamycin
- First generation cephalosporins
- Pasteurella spp. are fastidious Gram negative rods that grow well on all types of differential and selective agars.
- True
- False
- All of the following organisms may be routinely recovered from cat bite wounds except:
- streptococci
- Staphylococcus aureus
- Neisseria spp.
- Klebsiella spp.
- Moraxella spp
References
- Moet GJ, Jones RN, Biedenbach DJ, et al. Contemporary causes of skin and soft tissue infections in North America, Latin America, and Europe: report from the SENTRY Antimicrobial Surveillance Program (1998-2004). Diagn Microbiol Infect Dis. 2007 Jan;57(1):7-13.
- Winn W, Allen S, Janda W et al, eds. Koneman's Color Atlas and Textbook of Diagnostic Microbiology. 6th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2006:219.
- Horner C, Fawley W, Morris K, et al. Escherichia coli bacteremia: 2 years of prospective regional surveillance (2010-2012). J Antimicrob Chemother. 2014 Jan;69(1):91-100.
- Francis DP, Holmes MA, Brandon G. Pasteurella multocida. Infections after domestic animal bites and scratches. JAMA. 1975 Jul 7; 233(1):42-45.
- Von Schroeder HP, Bell RS. Pasteurella multocida osteomyelitis: An unusual case presentation. Can J Infect Dis. 1996 Mar;7(2):137-139.
- Chodakewitz J, Bia FJ. Septic arthritis and osteomyelitis from a cat bite. Yale J Biol Med. 1988 Nov-Dec;61(6):513-518.
- Kofteridis DP, Christofaki M, Mantadakis E, et al. Bacteremic community-acquired pneumonia due to Pasteurella multocida. IJID. 2009; 13(3): 81-83.
- Mandell GL, Bennett JE, Dolin R. Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases. 7th ed. Philadelphia, USA: Churchill Livingston Elsevier;2010:2939-2941.
- Casey AC, Greenspoon JS, Lagasse LD. Pasteurella multocida bacteremia in a patient with ovarian cancer and chemotherapy-induced neutropenia. Infect Dis Obstet and Gynecol. 1995;3(5):205-209.
- Brougui P, Raoult D. Endocarditis due to rare and fastidious bacteria. Clin Microbiol Rev. 2001 Jan;14(1):177-207.
- Armstrong GR, Sen RA, Wilkinson J. Pasteurella multocida meningitis in an adult: case report. J Clin Path. 2000 May;53(5):234-235.
- Baskar B, Desai SP, Parsons MA. Postoperative endophthalmitis due to Pasteurella multocida. Br J Ophthalmol. 1997 Feb;81(2):172-173.
- MacKay K, Brown L, Hudson F. Pasteurella multocida peritonitis in peritoneal dialysis patients: beware of the cat. Perit Dial Int. 1997 Nov-Dec;179(6):608-610.
- Mortensen JE, Giger O, Rodgers GL. In vitro activity of oral antimicrobial agents against clinical isolates of Pasteurella multocida. Diagn Microbiol Infect Dis. 1998 Feb;30(2):99-102.
- Lion C, Lozniewski A, Rosner V, et al. Lung abscess due to beta-lactamase-producing Pasteurella multocida. Clin Infect Dis. 1999; 29(5):1345-1346.
- Versalovic J, Carroll K, Funke G, et al, eds. Manual of Clinical Microbiology. 10th ed. Washington DC, USA: ASM Press; 2011:583.
- Goldstein EJ, Citron DM, Richwald GA. Lack of in vitro efficacy of oral forms of certain cephalosporins, erythromycin, and oxacillin against Pasteurella multodcida. Antimicrob Agents Chemother. 1988 Feb;32(2):213-215.
- CLSI. Methods for Antimicrobial Dilution and Disk Susceptibility Testing of Infrequently Isolated or Fastidious Bacteria; Approved Guideline – Second Edition. CLSI document M45-A2. Wayne, PA: Clinical and Laboratory Standards Institute; 2010.
- Kristinsson, G. Pasteurella multocida infections. Pediatr Rev. 28:472-473.
Authors
2014
Edward J. Yoon, MD, FCAP
James E. Kirby, MD, D(ABMM), FCAP
Beth Israel Deaconess Medical Center
Answer Key
- Beta-lactam/beta-lactamase inhibitor combinations (a).
- False (b).
- Klebsiella spp. (d).
Excerpted for Case of the Month by:
Thomas Kirn, MD, PhD, FCAP
Clinical Pathology Education Committee
Assistant Professor, Robert Wood Johnson Medical School
This material was originally released as 2015 CPIP J Case 10: Microbiology - A Classic Case of Pasteurella multocida (SAM eligible)